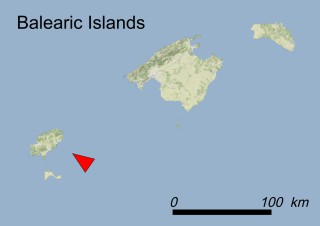
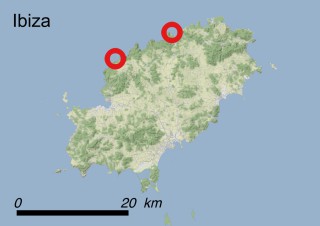
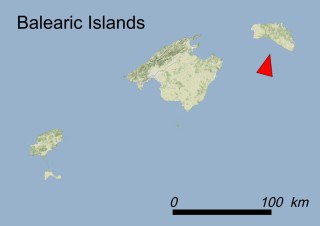
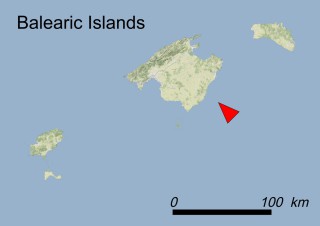
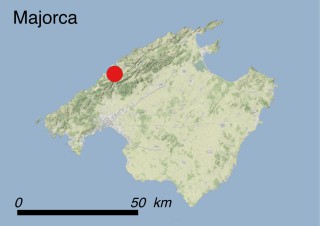
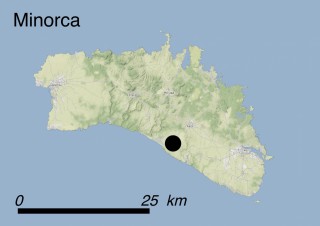
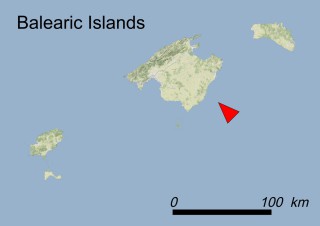
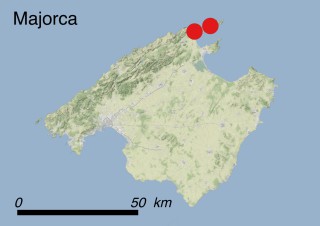
Where is it found ?
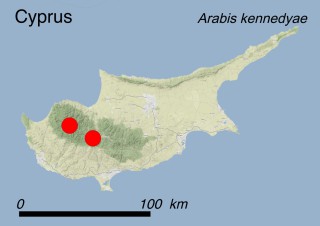
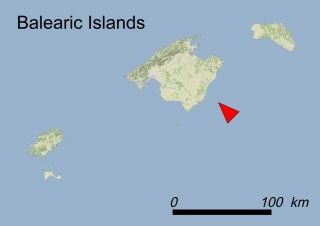
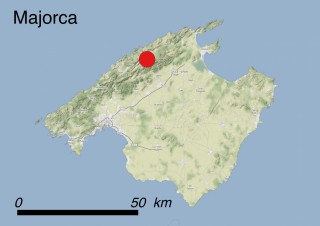
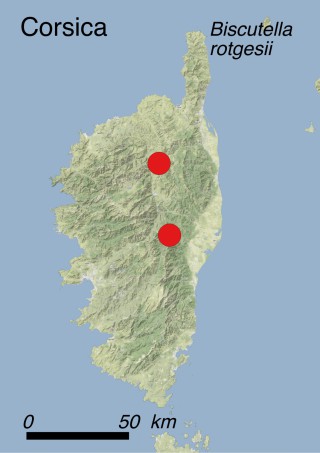
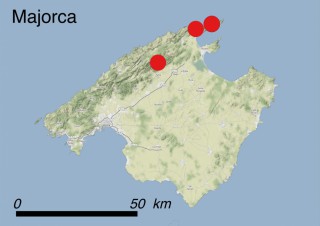
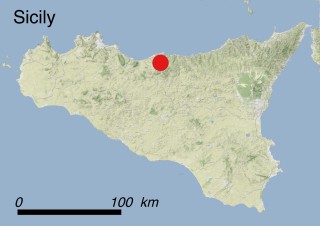
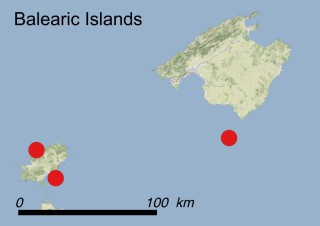
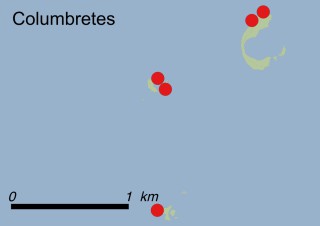
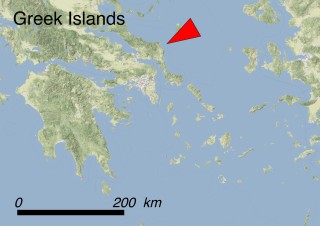
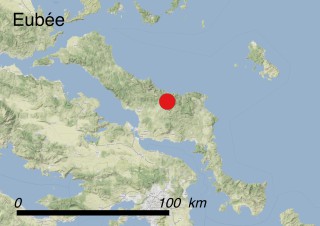
This species is mostly represented by small and scattered populations colonizing the screes and the sea-cliffs (habitats: 13.1 Sea cliffs and rocky offshore islands and 3.8.3: Spiny Mediterranean heaths - phrygana, hedgehog-heats and related coastal cliff vegetation) of some Balearic Islands and the Valencian Community (Eastern Spain). It grows on the satellite islets of Ibiza and Cabrera, in the Columbretes archipelago (province of Castellón, Valencian Community), and on one small islet (the ‘Illot de la Mona’ or ‘Escull del Cap de Sant Antoni’) situated just off the coast of the Cape of Sant Antoni (province of Alicante, Valencian Community as well). A few isolated individuals also occur on the mainland (NE of Alicante and harbour of Castellón de la Plana) as well as on the major Balearic islands (i.e. Western Ibiza). As for the Columbretes, two native populations remain on Ferrera and Foradada islands, while an artificial one has been reintroduced in the 1990s at lla Grossa. Some plants have been also introduced onto Es Pantaleu islet located just 180 m far from the western coast of Majorca.
How to recognise it ?
Medicago citrina is a small tree or round-shaped shrub reaching 2 m in height, with an erect single or multi-stemmed trunk. It has alternate, compound leaves composed of three leaflets. Flowers are lemon yellow in color, initially forming dense bunches which become sparser as the fruits start to mature. The plant flowers during late winter and early spring and the fruits form spiral-shaped pods. Some differences in morphological characters have been found amongst the 3 main population groups (Columbretes, Balearic Islands, and Northeastern Alicante).
Interesting facts
The species dominates the local endemic plant communities on the coastal cliffs where it occurs. Its seeds are thought to be dispersed by seabirds or other migratory animals. This species always grows at low altitudes on basic outcropping rocks, mostly limestones except for the Columbretes population, which grows on volcanic rocks. Pollination is performed by a wide range of insects, mainly hymenopterans in the Balearic islands and NE Alicante whereas in the Columbretes islands the main pollinators are hoverflies (Syrphidae, Diptera). The germination rate seems to increase when the seeds pass through the digestive tract of animals, but this condition is not strictly necessary for seedling germination. Natural populations are mainly formed by adult, old plants, showing a low proportion of young individuals, but the species forms a long-term soil seed bank which ensures a quick regeneration after the death of the parent plants. Although M. citrina was treated in the past as a subspecies or variety of M. arborea, several caryological and genetic studies pointed out recently that M. citrina is an hexaploid species (2n=48), well separated from the Eastern Mediterranean tetraploids M. arborea and M. strasseri (both 2n=32).
Why is it threatened ?
The species is considered Endangered (EN) based on IUCN Red List Criteria criterion C1. More in detail, the only 4 subpopulations of M. citrina have an AOO of 9 km2 when using a 1×1 km grid. Additionally, a strong decline of the species has been recently recorded: while 3195 plants were counted during the global census of 2008, there are currently only ca. 2500 remaining living adult individuals. The main current threat is represented by ongoing severe attacks of the alien scale insect Icerya purchasi (threat 8.1.2: Invasive non-native/alien species - Named species), which have severely affected the Columbretes Islands in 1996-97 and 2013-2015, causing respectively the reduction of 40% and close to 85% of the individuals of M. citrina populations. The same plague affected some Balearic populations in 2001, but showed lower effects. The decline within the Columbretes in 2013-15; from 1000 estimated individuals in 2008 to 166 in 2015, with less than 50 remaining healthy adult plants in 2016, was preceded by a strong weakening of adults and massive death of juvenile and seedling plants caused by extreme drought events in 2013-14 (threat 11.2: Drought). By the mid-2000s the population on Illot de la Mona was also affected by the same cochineal insect but showed minor damages. The two individuals living very close to the shoreline terrace of the Sant Antoni cliffs and representing the only native plants found on the mainland died by 2008, too. After the massive death of plants in the Ferrera island in 1997, further erosion of the volcanic soil caused by marine storms (threat 11.4: Storms & flooding) has reduced local habitat availability. Minor attack episodes by Cuscuta approximata (threat 8.2.2: Problematic native species/diseases - Named species) have been occasionally recorded in the Columbretes, but had a significant impact only on seedlings planted to reinforce the artificial population of Illa Grossa. In Illot de la Mona and on the Sant Antoni limestone cliffs, a part of the available rock crevices have been occupied by the invasive alien plant Opuntia ficus-indica (8.1.2), whereas this invasive plant has been mostly eradicated in the Columbretes since 1987. A new artificial population in NE Alicante was partially affected in 2015 by a nearby forest wildfire (threat 7.1.1: Fire and fire suppression - Increase in fire frequency/intensity), but further wildfire events seem to be almost improbable in the near future. Although the sites holding new Valencian populations are labelled in order to improve public awareness, occasional minor damages caused by trampling by tourists can be expected (threat 6.1: recreational activities). The ancient population of Illa Grossa became extinct by/during the 1960s, after a continuous decline trend from the 19th to the mid-20th century as a combined result of wood harvesting as fuel resource (threat 5.3.1: Logging & wood harvesting - Intentional use subsistence/small scale (species being assessed is the target), grazing due to introduced farm animals (threat 2.3.2: Small-holder grazing, ranching or farming) and rabbits (8.1.2), and the competition of Opuntia ficus-indica (8.1.2). After human abandonment of the Columbretes in the 1970s, the survival of introduced rabbits represented a severe problem until their total eradication, performed by the Valencian Wildlife Service in 1986-87. Rabbits also have affected some of the islets around Ibiza like S’Espartar. Apparently, the Balearic populations have not experienced a population decline in recent years. However, the lack of a detailed census does not allow confirming this demographic trend.
What has been done to protect it ?
Legally: The species is protected in the Valencian region since 1985, and for the whole Spanish territory since 1990. Currently it is included in the Spanish Catalogue of Threatened Species, being listed in Annex I of the Royal Decree 139/2011 as ‘Vulnerable’, a status that does not correspond to the IUCN’s homonym but guarantees strict in situ and ex situ protection of the plant, as well as its natural habitat. Medicago citrina is strictly protected under the same legal category (Vulnerable) in the Valencian Catalogue of Threatened Plant Species, listed in the Annex I of the regional Decree 70/2009 and Order 6/2013.
In situ: All the Valencian populations are subject to regular census and monitoring performed by the regional Wildlife Service, although no detailed demographic study has been made due to the extreme difficulty to access the three native populations. On the Columbretes, M. citrina occurs within a regional Nature Reserve created in 1988, and the two islets including native populations (Ferrera and Foradada) are additionally protected as Valencian Plant Micro-reserves (hereafter, PMRs) since 1998. Both sites additionally have the special status of Integral Reserves, so the access to the populations on the two islands is strictly prohibited apart from scientific research and conservation monitoring purposes. A new population was created in the 1990s in Illa Grossa. Both management plans of the Nature Reserve and the PMRs include specific actions to conserve the species. Since 1996, actions of biological control on the cochineal Icerya purchasi have been carried out by the regional Wildlife Service, by releasing adults of the predator ladybug Rodolia cardinalis. The main host plant of Icerya purchasi, the prickly pear cactus Opuntia ficus-indica, was almost completely eradicated in 1987-1990. On ‘Illot de la Mona’, approximately 40 plants grow within a plant micro-reserve, itself within the boundaries of El Montgó Nature Park, protected since 1987. The management plan of this PMR was approved in 2001, including actions for the in situ conservation of M. citrina. A new artificial population has been planted and reinforced in the nearby PMR ‘Cap de Sant Antoni’ since 2009. Recently two new populations have been planted in coastal localities on the mainland in NE Alicante and both sites are included within 2 PMRs. The aforementioned actions in the Valencian Community have been co-financed by several LIFE projects, i.e. LIFE93 NAT/E/000766, LIFE93 NAT/E/011100 and LIFE95 NAT/E/000856 ‘Setting up the plant micro-reserves network and acquisition of sites of priority botanical interest in the Valencian Community’ (1994-1999), and LIFE99 NAT/E/006417 ‘Conservation of priority habitats in the Valencian Community’ (1999-2003) to conserve the endangered flora and habitats of the Valencian Community, and afterwards supported with own and co-financed funds from the EU. All the small Balearic Islands are protected as Natural Areas of Special Interest under the Parliament of the Balearic Islands Law 1/1991. Some of these populations occur within the National Park of Cabrera. The Botanical Garden of Sóller has a program for the islets around Cabrera, including re-introduction and monitoring. Conservation introductions have been made on the islets of Es Pantyaleu and Na Redona. It is intended that these measures will also be applied to the islets of Ibiza. In 2015 rabbits had been definitely eradicated on the small island of S’Espartar (Ibiza). In addition, all the Valencian and Balearic sites holding native populations of M. citrina are included in Natura 2000 sites.
Ex situ: This plant is cultivated and seeds stored in the Centre for Forestry Research and Experimentation of the Valencian Community (CIEF), in the Botanical Gardens of Valencia and Sóller (Majorca) and in the Instituto Murciano de Investigación y Desarrollo Agropecuario (IMIDA) of Murcia. Production of new plants in the Columbretes is made in situ on Illa Grossa. The CIEF and the plant nursery of the Natural Park El Montgó maintain an orchard to produce the seeds for the ongoing ex situ activities of Illot de la Mona, and new plants are regularly produced to ensure the creation of new populations in NE Alicante. The Botanical Garden of Sóller provides plants for experimental plantations in the Balearic Islands. Due to its rapid growth and palatability, this species was proposed to be used as forage, and ex situ collections of living plants were established in several centers of the Spanish network of Agronomic Research Institutes in the provinces of Madrid, Murcia, Valencia and Zaragoza.
What conservation actions are needed ?
The priority is to control the attacks of the scale insect Icerya puchasi. The biological control is done by releasing its natural predator, the ladybug Rodolia cardinalis, but for the most accessible plants in the case of the Columbretes (e.g. reintroduced population of Illa Grossa) this action should be supported with water supply during the summer season. The control of the invasive plant Opuntia ficus-indica on Illot de la Mona is currently performed by the cactus cochineal Dactylopius opuntiae; the removal of died individuals can be recommended, in order to allow rock crevices to be recolonized by M. citrina and other native species. No effective control measures need to be undertaken to stop the occasional attacks of Cuscuta approximata in the Columbretes. Restoration of microsites affected by marine erosion in the Ferrera island should be proposed if the affected surface increases in the future. Preventive actions around the PMR ‘Cap de Sant Antoni’ should be made to reduce the risk of future wildfire damages. The creation of safe new populations in NE Alicante, as well as the reinforcement on Illa Grossa must be achieved during the next years. Due to the extreme difficulty to carry out a detailed demographic monitoring in the Columbretes and the Illot de la Mona sites, some of the new artificial populations should be studied, at least when the introduced plants will be adults. In addition, the Balearic populations should be surveyed in order to estimate their demographic trend.
Scientific coordination
Dr. Emilio Laguna, CIEF-Wildlife Service, Generalitat Valenciana, Valencia.
Dr. Llorenç Sáez, Authonomous University of Barcelona, Barcelona.
Co-authors:
Dr. Maurici Mus, University of Balearic Islands, Palma de Mallorca.
Dr. Ana Juan, University of Alicante, Alicante.
Dr. Manuel B. Crespo, University of Alicante, Alicante.
Photos
Dr. Emilio Laguna, CIEF-Wildlife Service, Generalitat Valenciana, Valencia.
Dr. Ana Juan, University of Alicante, Alicante.
Josep Lluis Gradaille, Jardí Botànic de Sóller, Sóller.
Additional references
Aguilella A., Fos S., Laguna E. (eds.). 2010. Catálogo Valenciano de Especies Amenazadas de Flora. 358 pp. Col. Biodiversidad nº 18. Conselleria de Medi Ambient, Aigua, Urbanisme i Habitatge. Generalitat Valenciana. Valencia.
Alomar G., Mus M., Rosselló J.A., 1997. Flora endèmica de les Balears. Consell Insular de Mallorca. Palma de Mallorca.
Bañares A., Blanca G., Güemes J., Moreno J.C., Ortiz S. (eds.) 2009. Atlas y Libro Rojo de la Flora Vascular Amenazada de España. Adenda 2008. Dirección General para la Biodiversidad- Sociedad Española de Biología de la Conservación de Plantas. Madrid.
Bibiloni G., Alomar G., Rita J., 1993. Flora vascular dels illots i addicions a la flora de Cabrera Gran. In Alcover, J.A., E. Ballesteros & J.J. Fornós (eds.): Història Natural de l’Arxipèlag de Cabrera 179-206. Monografies de la Societat d'Història Natural de les Balears, núm. 2. Ed. Moll & CSIC. Palma de Mallorca.
Boira H., Carretero J.L., 1987. Flora vascular de las islas Columbretes. In: Alonso L.A., Carretero J.L., García-Carrascosa M. (eds.): Islas Columbretes: contribución al estudio de su medio natural: 109-128. Monografias COPUT, n°5, Generalitat Valenciana. Valencia.
Boscaiu M., Riera J., Estrelles E., Güemes J.,1997. Números cromosomáticos de plantas occidentales, 751-776. Anales del Jardin Botanico de Madrid 55: 430-431.
Calduch M., 1992. Plantes vasculars del quadrat UTM 31SCE01. Els Columbrets. ORCA: Catàlegs florístics locals, 4. Barcelona.
Carretero J.L., Boira H., 1987. La vegetación de las islas Columbretes. In: Alonso L.A., Carretero J.L., García-Carrascosa M. (eds.): Islas Columbretes: contribución al estudio de su medio natural: 129-153. Monografias COPUT, n°5, Generalitat Valenciana. Valencia.
Chebbi H., Ríos S., Pascual-Villalobos M.J., Correal E., 1994. El grupo Medicago arborea en la cuenca Mediterránea: II. Comportamiento frente a la sequía. Pastos 24: 177-188.
Chebbi, H., Pascual-Villalobos M.J., Cenis J.L., Correal E., 1995. Caractérisation morphologique et moléculaire des espèces ligneuses du genre Medicago. Fourrages 142: 191-206.
CIEF-SVS, 2013. Conservación de Medicago citrina en la Comunidad Valenciana. 11 p. Informe Técnico 09/2013. Centro para la Investigación y Experimentación Forestal (CIEF) - Servicio de Vida Silvestre (SVS). Generalitat Valenciana, Valencia. Available at: http://www.citma.gva.es/web/biodiversidad/conservacion-de-medicago-citrina-en-la-comunitat-valenciana
Contandriopoulos J., Cardona M.A., 1984. Caractére original de la flore endémique des Baléares. Botanica Helvetica 94(1): 103-132.
Crespo M.B., Juan A., Mus M., Laguna E., 2005. Medicago citrina. In: Montmollin B., Strahm W. (eds.): The Top 50 Mediterranean Island Plants. Wild plants at the brink of extinction, and what is needed to save them: 26-27. Mediterranean Island Plant Group, IUCN. Gland & Cambridge
Crespo M.B., Juan A., Alonso M.A., Martínez-Flores F. Martínez-Azorín M. 2007. Biodiversidad vegetal del Parque Nacional de Cabrera: Biología de la conservación y diseño de estrategias de gestión de endemismos vasculares insulares. In: OAPN: Proyectos de investigación en parques nacionales 2003-2006:129-148. Organismo Autónomo de Parques Nacionales (OAPN), Ministerio de Medio Ambiente, Madrid.
Draper, D. 2013. Medicago citrina. The IUCN Red List of Threatened Species 2013: e.T61653A12533350. http://dx.doi.org/10.2305/IUCN.UK.2011-1.RLTS.T61653A12533350.en Available at http://www.iucnredlist.org/details/61653/0
Fabregat C., Laguna E., 2013. From fire to nature: evolution, restoration and conservation of the Columbretes Islands flora. In Cardona E., Estaún I., Comas M., Fraga P. (eds.): Islands and Plants: preservation ands understanding of flora on Mediterranean Islands: 297-308. Consell Insular de Menorca. Maó, Menorca.
Fabregat C., Pérez-Rovira P., Mestre E., del Señor X., Castañer V., Viñes M., Tena V., 2011. Conservación de especies amenazadas en la Reserva Natural de las Islas Columbretes (Castellón): evaluación de las poblaciones de Fumaria munbyi Boiss. & Reuter, Medicago citrina (Font Quer) Greuter y Reseda hookeri Guss. Poster communication to the V Congreso de Biología de Conservación de Plantas, Es Mercadal (Menorca), 28th. Sept-1st Oct. 2011. Available at: http://www.uibcongres.org/imgdb//archivo_dpo11406.pdf
Ferrer-Gallego P.P., Ferrando I., Gago C., Laguna E. (eds.) 2013. Manual para la conservación de germoplasma y el cultivo de la flora valenciana amenazada. 2ª ed. 252 pp. Colección Manuales Técnicos Biodiversidad, 3. Conselleria d’Infraestructures, Territori i Medi Ambient. Generalitat Valenciana. Valencia.
Ferrer-Gallego P.P., Ferrando I., Escribá M.C., Albert F.J., Navarro A., Martínez V., Hurtado A., Laguna E. 2013 El Banco de germoplasma de flora silvestre valenciana: la colección CIEF (1999-2012). Chronica Naturae 3: 76-82.
Ferrer-Gallego P.P., Ferrando I., Albert F.J., Martínez V., Escribá M.C., Navarro A., Laguna E., 2014 Conservación y distribución de las accesiones del Banco de Germoplasma de Flora Silvestre Valenciana en la colección CIEF. Cuadernos de Biodiversidad 46: 9-18. DOI: http://dx.doi.org/10.14198/cdbio.2014.46.02
González-Andrés F., Chávez J., Montañés G., Ceresuela J.L., 1999. Characterisation of woody Medicago (sect. Dendrotelis) species, on the basis of seed and seedling morphometry. Genetic Resources and Crop Evolution 46: 505-519. DOI: http://dx.doi.org/10.1023/A:1008732400424
Juan A., 2002. Estudio sobre la morfología, variabilidad molecular y biología reproductiva de Medicago citrina (Font Quer) Greuter (Leguminosae). Bases para su conservación. PhD Thesis (unpublished). Universidad de Alicante. Alicante.
Juan A., Crespo M.B., 1999. Comportamiento fitosociológico de Medicago citrina (Font Quer) Greuter (Leguminosae), endemismo Mediterráneo-Iberolevantino. Acta Botanica Malacitana 24: 221-229.
Juan A., Crespo M.B., 2001. Producción de flores y frutos en las poblaciones ibicencas de Medicago citrina. In: Crespo M.B., Ríos S., Juan A. (eds.): Actas de la XLI Reunión Científica de la Sociedad Española para el Estudio de los Pastos (SEEP): 275- 280. Universidad de Alicante & Sociedad Española de Estudio de los Pastos (SEEP). Alicante.
Juan, A. & M.B. Crespo. 2009. Scanning electron microscope characters and their systematic significance within Medicago L. sect. Dendrotelis (Fabaceae: Trifolieae). Feddes Repertorium 120 (3-4): 158-168. DOI: http://doi-dx.org/10.1002/fedr.200811198.
Juan A., Crespo M.B., Alonso M.A., 2009. Medicago citrina (Font Quer) Greuter. In Bañares, A., G. Blanca, J. Güemes, J.C. Moreno & S. Ortiz (eds.): Atlas y Libro Rojo de la Flora Vascular Amenazada de España: Addenda 2008: 54-55. Ministerio de Medio Ambiente, Medio Rural y Marino. Madrid.
Juan A., Crespo M.B., Ríos S., 1999. Medicago citrina (Font Quer) Greuter (Leguminosae): variabilidad morfológica, ecología y estado actual de sus hábitats. In: SEEP (ed.): Actas de la XXXIX Reunión Científica de la Sociedad Española para el Estudio de los Pastos (SEEP): 87-91. SEEP. Almería.
Juan A., Crespo M.B., Ríos S., 2003. Remarks on Medicago citrina (sect. Dendrotelis, Leguminosae). Flora Mediterranea 13: 303-316.
Juan A., Crespo M.B., Cowan R.S., Lexer C., Fay M.F., 2004. Patterns of variability and gene flow in Medicago citrina, an endangered endemic of islands in the western Mediterranean, as revealed by amplified fragment length polymorphism (AFLP). Molecular Ecology 13: 2679-2690. DOI: http://doi.dx.org/10.1111/j.1365-294X.2004.02289.x
Kell S.P., Laguna E., Iriondo J.M., Dulloo M.E., 2008. Population and habitat recovery techniques for the in situ conservation of plant genetic diversit”. In: Iriondo J.M., Maxted N., Dulloo M.E. (eds.): Conserving Plant Genetic Diversity in Protected Areas. Population management of Crop Wild Relatives: 124-168.CABI. Wallingford. DOI: http://dx.doi.org/10.1079/9781845932824.0124
Klemmer K., 1996. Las islas Columbretes die Schlangeninseln ohne Schlangen. Natur und Volk 91(2): 39-47
Laguna E., (coord., ed.). 1994. Libro de la flora vascular rara, endémica o amenazada de la Comunidad Valenciana. 274 p. Consellería de Medio Ambiente. Generalitat Valenciana. Valencia
Laguna E., (coord., ed.). 1998. Flora rara, endémica o amenazada de la Comunidad Valenciana. 445 p. Consellería de Medio Ambiente. Generalitat Valenciana. Valencia.
Laguna E., 2004. The plant micro-reserve initiative in the Valencian Community (Spain) and its use to conserve populations of crop wild relatives. Crop Wild Relative, 2: 10-13.
Laguna E., 2005. Microrreservas, conservación ‘in situ’ y planes de recuperación de flora amenazada. Recursos Rurais 2: 81-90
Laguna E., 2011. Re-introduction of Spanish moon trefoil in Illa Grossa, Columbretes Islands, Spain. In: Soorae P.S. (ed.): Global Re-Introduction Perspectives: 2011. More case studies around the globe: 239-243. IUCN Re-Introduction Specialist Group. Gland & Abu-Dhabi.
Laguna E., Jiménez J.L., 1995. Conservación de la flora de las islas Columbretes. Ecologia Mediterranea 21(1-2): 325-336.
Lefi E., Gulías J., Cifre J., Ben-Younes M., Medrano H., 2004. Drought effects on the dynamics of leaf production and senescence in field-grown Medicago arborea and Medicago citrina. Annals of Applied Biology144: 169-176. DOI: http://dx.doi.org/10.1111/j.1744-7348.2004.tb00330.x
Lefi E., Conesa M.A., Cifre J., Gulías J., Medrano H., 2012. Dry matter allocation in Medicago arborea and Medicago citrina in response to drought and defoliation. Crop and Pasture Science 63(2): 179-189. http://dx.doi.org/10.1071/CP12036
Lo Bianco M., 2014. New shape and texture descriptors for an improved germplasm characterization of the most representative Mediterranean flora. PhD Thesis. Università degli Studi di Cagliari, Cagliari. Available at: http://veprints.unica.it/1087/1/PhD_Thesis_LoBianco.pdf
Lo Bianco M., Ferrer-Gallego P.P., Grillo O., Laguna E., Bacchetta G., 2015. Seed image analysis provides evidence of taxonomical differentiation within the Medicago L. sect. Dendrotelis (Fabaceae). Systematics and Biodiversity 13 DOI: http://dx.doi.org/10.1080/14772000.2015.1046968
Pérez Bañón, C. 2000. Biologia de dos Sirfidos (Diptera: Syrphidae) de los ecosistemas insulares de la Comunidad Valenciana: aspectos de la relacion Sirfido-Planta. PhD Thesis (unpubl.). Universidad de Alicante. Alicante.
Pérez-Bañón C., Juan A., Petanidou T., Marcos-García M.A., Crespo M.B., 2003. The reproductive ecology of Medicago citrina (Font Quer) Greuter (Leguminosae): a bee-pollinated plant in Mediterranean islands where bees are absent. Plant Systematics and Evolution 241: 29-46. DOI: http://dx.doi.org/10.1007/s00606-003-0004-3
Ríos S., Laguna E., 2010. Adaptations for the survival of perennial legumes in Western Mediterranean regions: Some promising native species of the Valencian Community (Spain). Options Méditerranéennes, Ser. A, 92: 9-23.
Rita J., Bibiloni G., 1993. La vegetació de Cabrera (Memòria del mapa de les comunitats vegetals). In: Alcover J.A., Ballesteros E., Fornós J.J. (eds.): Història Natural de l'Arxipèlag de Cabrera: 207-255. Monografies de la Societat d'Història Natural de les Balears, núm. 2. Ed. Moll & CSIC. Palma de Mallorca.
Rita J., Bibiloni G., 2013. The flora of the islets of the Balearic Islands. In Cardona E., Estaún I., Comas M., Fraga P. (eds.): Islands and Plants: preservation ands understanding of flora on Mediterranean Islands: 309-322. Consell Insular de Menorca. Maó, Menorca
Robledo A., Ríos S., Correal E., 1993. El grupo Medicago arborea en la cuenca Mediterránea: Origen, distribución y morfologia. Pastos 23(2): 325-336.
Rosato M., Rosselló J.A., 2009. Karyological observations in Medicago section Dendrotelis (Fabaceae). Folia Geobotanica 44: 423-433. DOI: http://dx.doi.org/10.1007/s12224-009-9048-7
Rosato M., Castro M., Rosselló J.A., 2008. Relationships of the woody Medicago species (Section Dendrotelis) assessed by molecular cytogenetic analyses. Annals of Botany 102: 15-22. DOI: http://dx.doi.org/10.1093/aob/mcn055
Rosato M., Galián J.A., Rosselló J.A., 2012. Amplification, contraction and genomic spread od a satellite DNA family (E180) in Medicago (Fabaceae) and allied genera. Annals of Botany 109: 773-782. DOI: http://dx.doi.org/10.1093/aob/mcr309
Sáez Ll., Rosselló J.A., 2001. Llibre Vermell de la Flora Vascular de les Illes Balears. Direcció General de Biodiversitat. Conselleria de Medi Ambient. Govern de les Illes Balears
Serra L., 2007. Estudio crítico de la flora vascular de la provincia de Alicante: Aspectos nomenclaturales, biogeográficos y de conservación. Ruizia 17: 1-1414.
Serra L., Pérez J., Izquierdo J., 2001. Medicago citrina (Font Quer) Greuter (Leguminosae) en la Península Ibérica. Anales del Jardin Botánico de Madrid 59(1): 158-159.
Sobrino E., Hervella A., Ceresuela J.L., Barbado A., Viviani A., De Andrés F., Tenorio J.L., 2000. Morfología y taxonomía de la Sección Dendrotelis del género Medicago (Fabaceae). Portugaliae Acta Biologica 19: 225-237.
Walter K.S., Gillett H.J. (eds). 1998. 1997 IUCN Red List of Threatened Plants. Compiled by the World Conservation Monitoring Centre. IUCN-Gland and Cambridge